OECD 443: Extended one-generation reproductive toxicity study (EOGRTS)
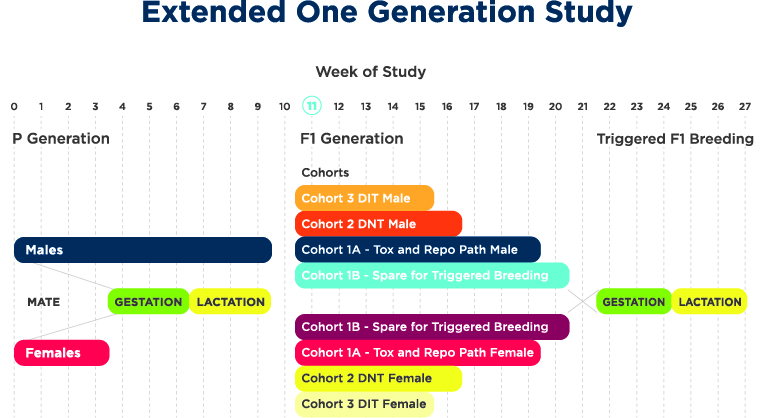
The objectives of the extended one generation study (OECD 443) are to determine the adverse reproductive and developmental effects that may occur as a result of pre- and postnatal chemical exposure as well as an evaluation of systemic toxicity in pregnant and lactating females and young and adult offspring.
This study evaluates reproductive performance, necropsy, organ weights, sperm motility/counts/morphology, histopathology and ovarian follicle counts of the offspring (F1 generation). The OECD 443 study is primarily conducted in rats via dietary or oral gavage routes of administration.
-
Molecular property and toxicity prediction using ACD/Percepta®
Modeling, Toxicology -
Toxic hazard estimation (ToxTree©)
Modeling, Toxicology -
EPA: Toxicity Estimation Software Suite (TEST) analysis (U.S.)
Modeling, Toxicology -
OECD QSAR toolbox
Modeling, Toxicology -
OECD 441: Hershberger assay
Toxicology
